Our developers and data engineers worked closely with the client’s teams to identify possible roadblocks and develop a solution that was agreeable. A few key challenges stood out:
Business units like Quality Assurance and Supply Chain were heavily dependent on data analysts to generate reports. This created a constant lag in accessing insights, slowing down the pace of decision-making for daily operations and leaving teams frustrated.
The organization’s most critical data was scattered across ERP, LIMS, MES, and regulatory systems. Since these platforms didn’t “talk” to each other, every analysis became scattered, often resulting in duplicated reports and incomplete views of the truth.
Non-technical teams had no straightforward way to explore or interact with data on their own. Every small query had to be routed via IT, stretching timelines, while also keeping business users from developing data confidence.
With no predictive analytics in place, the company could only respond after issues had already surfaced. This reactive approach made it difficult to detect process deviations or compliance risks early, which in turn affected both production timelines and regulatory confidence.
After sketching the problems, our team suggested building a modern augmented analytics architecture. Through focused discussions with their stakeholders, we designed a system that unified data, enabled self-service, and brought predictive intelligence into their daily ops.
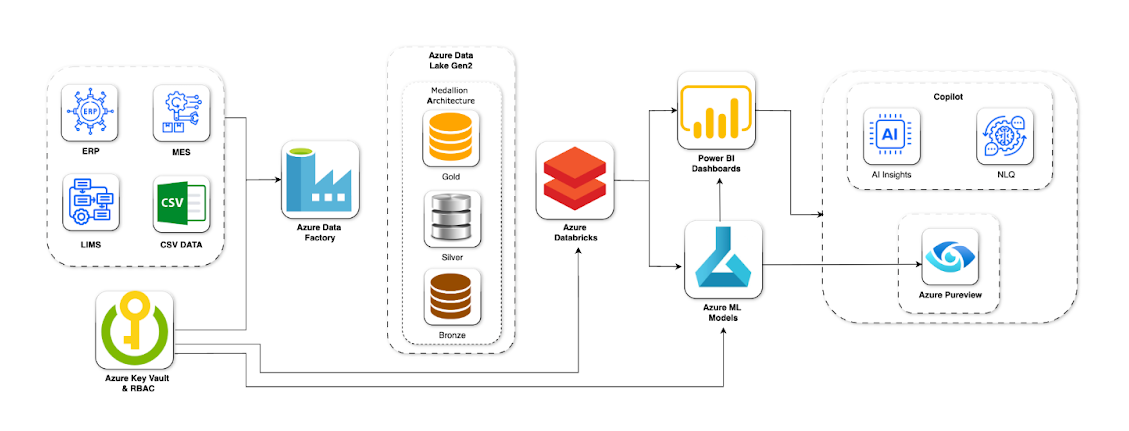
We used Azure Data Factory to connect ERP, LIMS, MES, and regulatory systems into a single data lake. To ensure quality, validation, and full traceability, we applied a medallion architecture (Bronze-Silver-Gold).
Power BI with Copilot was introduced as the analytics layer, offering AI-powered dashboards and natural language queries. Business teams could now access auto-generated insights, detect anomalies, and forecast trends in real time.
Using Azure ML, we developed machine learning models for predictive quality control and production efficiency. These models flagged potential deviations early, reducing batch failures and compliance risks.
Role-based access control (RBAC) was enforced to secure sensitive data in line with MHRA/EMA standards. We also implemented data lineage, audit trails, and metadata management for complete transparency.
Interactive self-service dashboards were built for Quality, R&D, and Supply Chain. To drive adoption, our team conducted workshops and training sessions, boosting confidence and data literacy across teams.
The solution was deployed on Microsoft Azure to meet GxP and pharma regulatory standards. Azure Key Vault and Managed Identities secured credentials, while Azure Monitor and Log Analytics enabled logging, auditing, and full traceability.
Azure Data Factory ingested structured and semi-structured data from ERP, LIMS, MES, and CSVs. All data was centralized in Azure Data Lake Gen2, organized through a Medallion architecture for quality and pipeline optimization.
Azure Databricks with PySpark handled scalable transformations and feature engineering. Custom rules standardized batch metadata and harmonized data types, while Azure ML models provided predictive insights on quality deviations and supply bottlenecks.
Power BI dashboards displayed real-time KPIs, quality trends, and process bottlenecks. With Copilot, complex analytics were explained in natural language, and users could generate reports through simple queries without IT support.
Azure Purview and Monitor ensured lineage, data refresh schedules, and audit logs. Compliance dashboards maintained validated records, keeping the system always ready for MHRA/EMA audits.
With self-service dashboards and AI-powered insights, business teams could generate reports in minutes instead of days. Reporting time was cut down by nearly 70%, giving stakeholders faster access to the information they needed.
Predictive analytics flagged production bottlenecks and potential quality issues before they escalated. This proactive approach improved operational efficiency by around 40%, reducing both downtime and costly rework.
Natural language query tools made analytics accessible to non-technical teams. Over 85% of business users adopted these features, easing the load on IT and fostering data-driven decision-making across departments.
Automated validation and traceable workflows streamlined compliance processes. The client reported audit preparation times reduced by nearly 60%, ensuring smoother readiness for regulatory reviews.
For the client, this journey wasn’t only about new tools or architectures; it was about freeing teams from problems and giving them ownership of their data. With custom dashboards, predictive insights, and stronger compliance, every department now works with more confidence.
Healthcare & Life Sciences
Europe
End to End Project Lifecycle Management
Briefly describe the challenges you’re facing, and we’ll offer relevant insights, resources, or a quote.

Business Development Head
Discussing Tailored Business Solutions
DataToBiz is a Data Science, AI, and BI Consulting Firm that helps Startups, SMBs and Enterprises achieve their future vision of sustainable growth.
DataToBiz is a Data Science, AI, and BI Consulting Firm that helps Startups, SMBs and Enterprises achieve their future vision of sustainable growth.




